When it comes to cell and gene therapy, setting the right price isn’t easy. As these therapies target unique and/or rare diseases with high unmet medical need, there aren’t any pricing benchmarks for companies to refer to. Expert Rainer Opgen-Rhein explain the importance of international referencing pricing for the successful launch of a cell or gene therapy.
Price benchmarks play an important role when setting prices for pharmaceutical products. This applies to setting prices for comparative therapies within a country (internal referencing) or setting prices for the same product in other countries. The latter is called international reference pricing (IRP).
In this article we will outline why this benchmark is so important to consider when launching a cell or gene therapy, and what tools can be used to succeed?
The importance of international reference pricing in the cell and gene therapy industry
As we stated in our previous article on the subject, IRP is a process in which national decision bodies compare cross-country prices in order to set or influence the price of a product in its own country. IRP can roughly be distinguished in two different ways on how prices in other countries are used.
Either it’s a hard stop – a strict maximum price set and regulated by an official law according to a specific formula. In other words, a formal application. Or the reference price is one of several elements in price setting – as such, there’s still room to negotiate the final price. We call this an informal application.
A fixed, or formal price, sets a clear threshold which companies cannot cross when setting prices. With informal international reference pricing, however, its importance depends on the specific situation. If a therapy area is very competitive with many treatment options and therefore already has set price benchmarks within a country, international prices typically play less of a role. But if there is little to no competition, external prices have more sway.
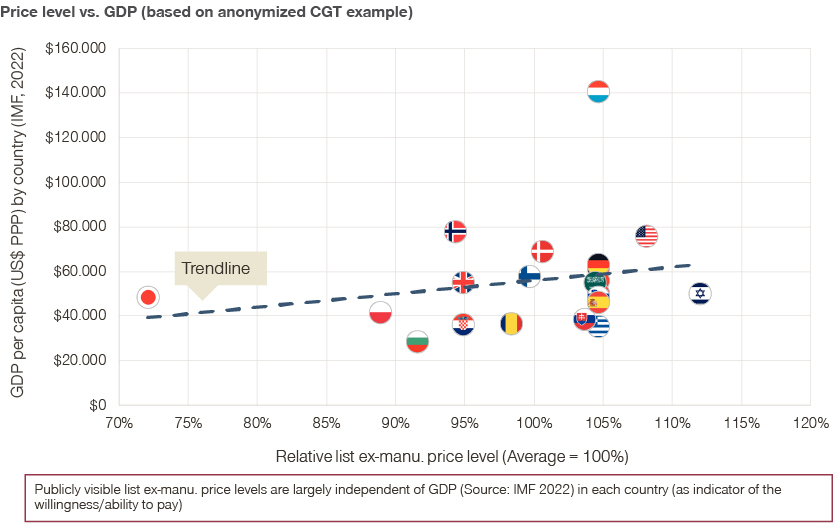
This is why international reference pricing plays an especially significant role in cell and gene therapy (CGT). CGTs tend to target orphan indications, which don’t currently face real competition. Often there are only therapies available to alleviate symptoms with prices far below the standard CGT price tag.
IRP, therefore, is more or less the only price benchmark available for CGTs. Yet, it remains that while IRP is constantly being adopted by new markets, incumbents are regularly introducing stricter and more challenging rules. Therefore, to successfully launch a CGT, a comprehensive preparation and global launch strategy is required.
The international reference pricing factors cell and gene therapy companies need to consider
As international reference pricing is widely adopted around the world, there are many different (and strict) rules, which make it more difficult to differentiate prices across countries, especially for CGTs without internal price benchmarks.

Given the importance of IRP, companies wishing to launch a cell or gene therapy need to conduct a detailed analysis of international referencing mechanisms for the specific product and consider IRP for their global launch strategy. While a narrow price corridor allows to minimize the impact of IRP, it might not reflect the willingness/ability to pay in individual countries, leading to less patient access to innovative treatments.

One viable way to differentiate list prices between countries to reflect country-individual ability and/or willingness to pay is – smart launch sequencing. This should be considered in the development of every global launch strategy.
Nevertheless, even launch sequence optimization might not be sufficient to differentiate prices sufficiently to allow patients access to innovative therapies.
Further differentiation can be achieved on the net price level, by using innovative contracting such as pay-for-performance or risk-sharing agreements.
How to successfully launch a cell and gene therapy considering international reference pricing
Taking into account these complications, launching a CGT requires careful preparation and the development of a comprehensive global launch strategy that considers the following:
- An assessment of country specific pricing and market access (P&MA) regulations, as well as within-country price potential on a list and net price level
- Using IRP assessment and international launch sequencing to coordinate a cross-country launch strategy
- Using innovative contracting to help differentiate prices
In our previous article we outlined the five-step strategic approach to international reference pricing. These steps also apply to CGT launches – from knowing the IRP regulations in each country to using an advanced analytics model to assess the IRP impact and optimize the launch strategy.
However, developing a global launch strategy once is not sufficient. The strategy has to be transferred to and also be implemented in specific markets, which includes preparing to negotiate with local authorities.
In addition, a global launch strategy is never static: It must be continually monitored and updated if any assumptions change and new information is available (e.g., achieved prices in these negotiations, achieved time-to-market, or other changes in the P&MA landscape).
Simon-Kucher IRP Genius
Our Simon-Kucher IRP Genius is a comprehensive IRP assessment solution allowing you to manage the potential impacts of international reference pricing. It contains a comprehensive IRP rule library that considers the specific regulations for each market. Our tool can analyze and identify the rules that apply to your specific CGT product and target market, to help you set the best prices for your company and patients.
If you are looking to launch a cell and/or gene therapy product but are worried about international reference pricing implications, contact our expert Rainer Opgen-Rhein.
To learn more about our software solutions and Simon-Kucher Engine, visit our website.
Further reads:
Cell & Gene Therapy: Curing the "Incurable"
Launch planning for cell & gene therapies
How Pharma can Overcome Barriers to CGT Diagnostic Testing
Value-based care toolkits for cell and gene therapies








